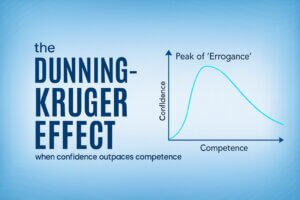
The Confidence Trap in Clinical Trials: When Knowing Just Enough Becomes Dangerous
When confidence outpaces competence in clinical research, the risks can be hidden…and costly for trial sponsors

When confidence outpaces competence in clinical research, the risks can be hidden…and costly for trial sponsors

When confidence outpaces competence in clinical research, the risks can be hidden…and costly for trial sponsors
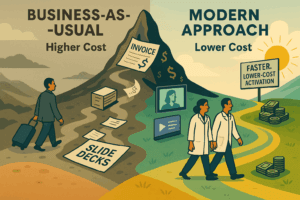
Clinical trials aren’t just more complex. They’re harder to manage, more expensive to run, and increasingly exposed to delay and disruption. Sponsors willing to take a different path can deliver faster, higher-quality trials at a lower cost.

I. Introduction: Two Conferences, One Reality This past week at DIA and BIO offered two very different views of the clinical research world. DIA felt

In clinical research, where accuracy, coordination, and compliance are non-negotiable, the greatest threat isn’t always a lack of knowledge. Sometimes, it’s the mistaken belief that

Clinical trials professionals face a host of challenges: for some, it’s the complexity in protocol design, for many it’s lagging enrollment rates, and for others it’s the site burden that’s inherent to change management.

The compounding benefits of effective training cannot be overstated. When clinical research associates (CRAs) and site staff are thoroughly prepared, they engage more deeply with the trial protocols, ask more insightful questions, and more proactively anticipate challenges.

I was recently invited to deliver a keynote presentation on embracing change. The audience was more than a 100 healthcare improvement and clinical trial professionals who had each committed to participate in a year-long collaborative.

The formal Formal adoption of the E6(R3) guideline introduces a proportionate and more modern risk-based approach to quality management. Clinical trial leaders must implement more effective training programs that address increased trial complexity and ensure compliance with the enhanced quality requirements.

What learning science has taught us about the drivers and predictors of change—and applying those to clinical research practice. This article was first posted November

Trial sponsors don’t miss timelines because they’re careless. They miss them because they’re human. Behavioral science calls it the planning fallacy.

We talk a lot about processes and platforms in clinical trials. But in the end, success comes down to understanding and supporting the needs of the people conducting the trial.

Four strategies for implementing this approach in clinical trial staff and site training. Brian S. McGowan, PhD, FACEHP, Chief Learning Officer and Co-Founder, ArcheMedX, Inc.
ArcheMedX helps life sciences and healthcare organizations to better equip, evaluate, and predict team and clinician performance
Get new original content and a hand-curated selection of the best life science articles from around the web directly in your inbox. Subscribe to The ArcheMedX Clinical Research Newsletter
See how ArcheMedX applies behavioral science to transform learning and generate actionable insights