Traditional Site Initiation Visits Waste Time and Money.
There is a Better Way.
With Ready by ArcheMedX, uncover risks sooner and upskill your clinical trial team to eliminate study delays.

Meet Ready for Clinical Trial Sponsors
The clinical trials learning and analytics platform that optimizes site initiation and helps you predict and improve study performance.
PRIORITIZE ACTIVATION, ACCELERATE ENROLLMENT, MITIGATE Risks
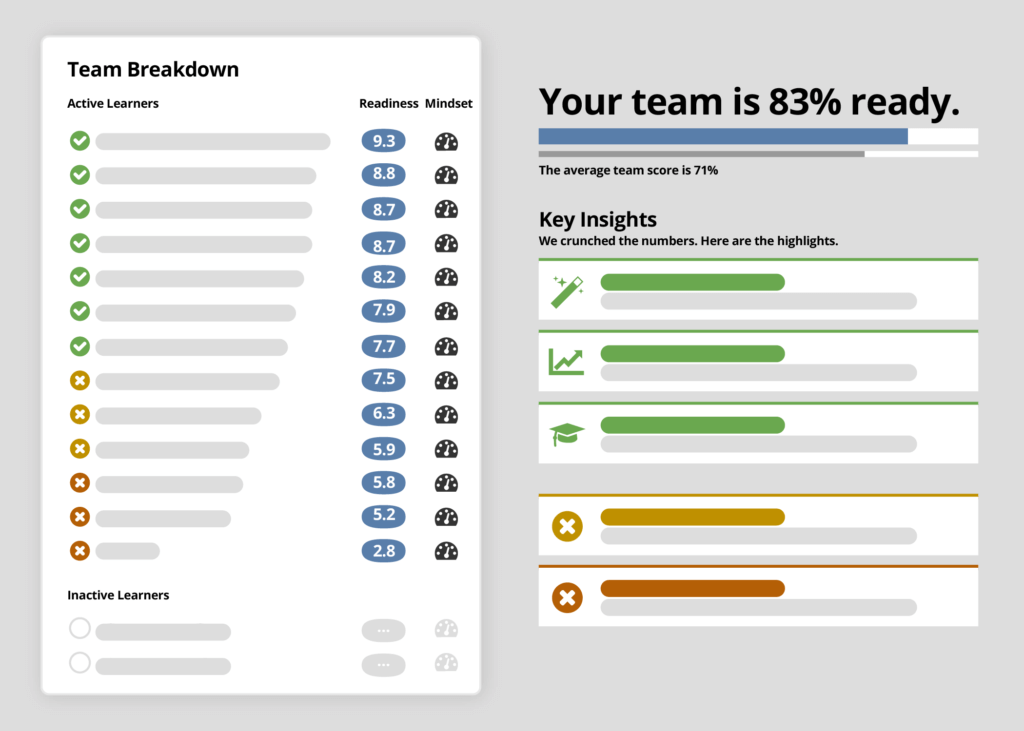
Ready enables you to to better understand which sites and teams are best prepared to start and effectively conduct the study. Utilize Ready’s real-time insights to prioritize site activation by front loading those sites best equipped to perform well and accelerate enrollment.
Drill down into more specific insights to prevent the most common sources of study delays and reduce time and costs spent resolving issues by identifying challenges far earlier in the trial. Then utilize these insights based on leading indicators of site readiness and risk to inform your risk-based monitoring coverage before critical delays and errors might appear in your traditional performance data.
BETTER EQUIP YOUR SITES AND TEAMS
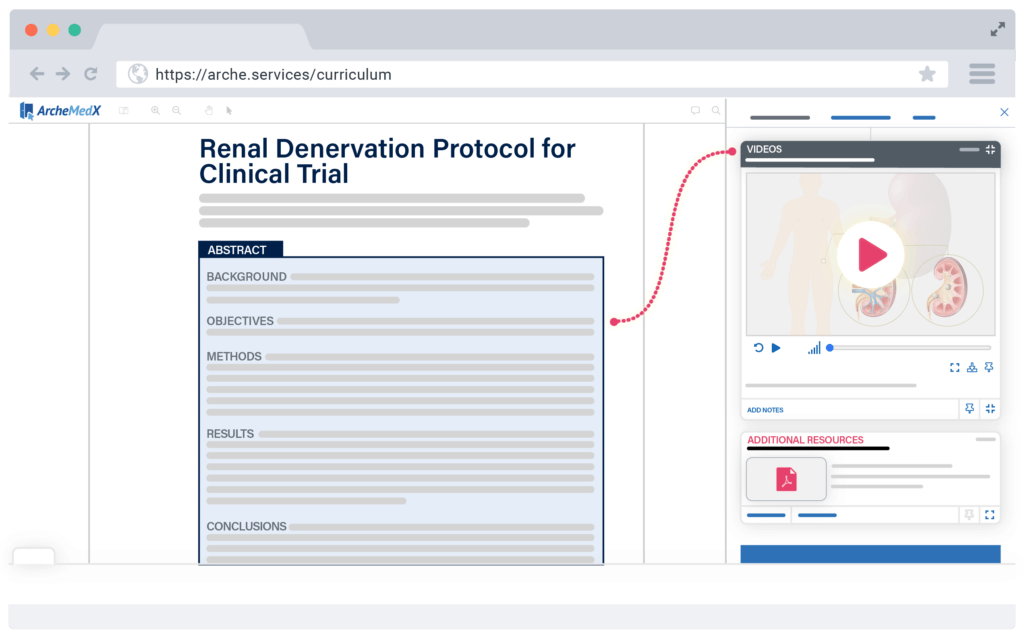
Re-imagine how you or your CRO runs site initiation and reduce the burden on your teams and sites. Train them on-demand ahead of a site visit and watch your actual study documents come alive as sites and trial teams are directed to focus and engage around key study objectives.
Eliminate the need to convert well crafted study documents into bad power point presentations or time consuming simulations, instead, let Ready enable a member of your team to quickly create a more effective, on-demand training experience tailored to individual study roles using your documents and Ready's step by step activity creation tools.
Then let Ready engage your sites and inform your understanding of who is or is not ready to conduct your trial as Ready assesses the confidence and capability of each participant. Ensure that your CRAs and trial team members are equipped with the study knowledge and skills they require to effectively conduct your trial.
REDUCE RISK AND DEVIATIONS
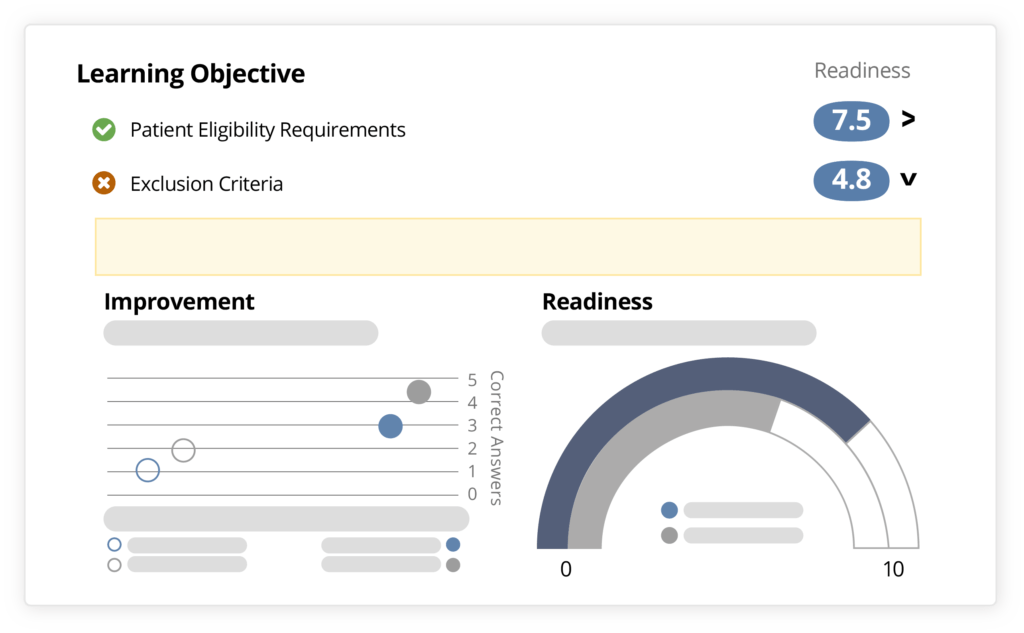
Ready reduces risk by predicting how individuals will apply knowledge and skills in real-world scenarios.
As you prepare sites to more effectively conduct your studies, discover the specific topic areas and study requirements where they are most likely to excel or inclined to struggle. Identify how confident your teams and sites truly are in conducting the most critical aspects of your study – so you can make more informed oversight and operational decisions.
By uncovering specific areas of confusion in your study content and potential low performers you can identify risks sooner and take proactive steps to avoid errors and delays.
OPTIMIZE STUDY PERFORMANCE
Keep sites and individuals engaged throughout the duration of the study and ensure they consume and can confidently apply new information, such as patient screening best practices or changes in standard of care.
Ready can automate a variety of remediation and retention programs, saving you valuable time by engaging and upskilling your team and sites automatically.
Enable the smooth roll out of protocol amendments and other communication. Utilize Ready's predictive insights to direct operational decisions sooner and inform risk-based monitoring (RBM) strategies.
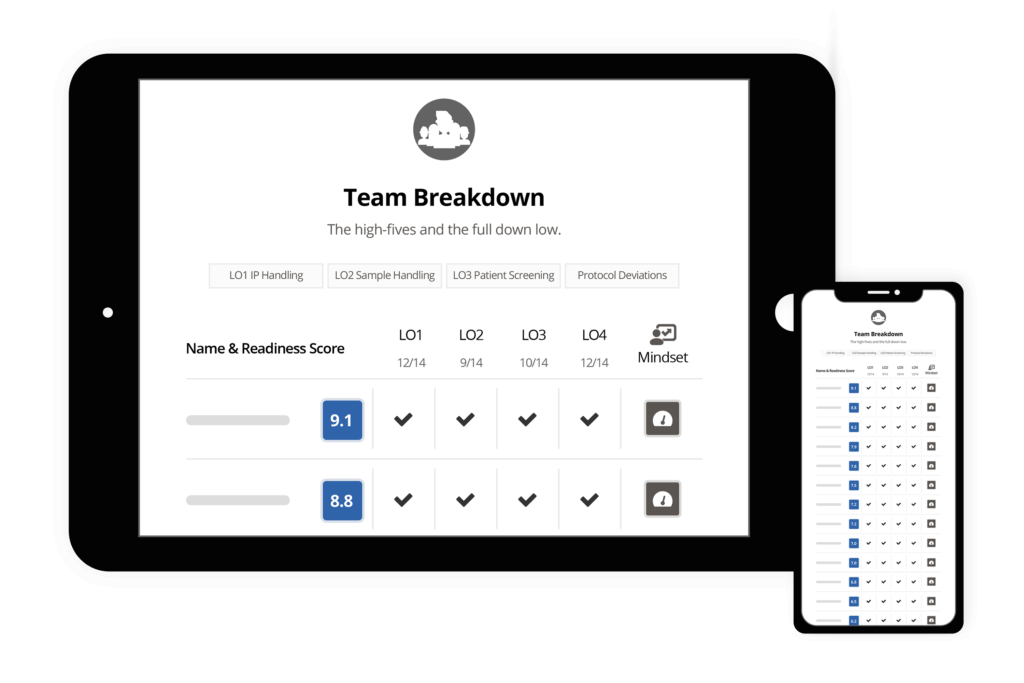
Use Ready to Optimize the Site Initiation Process
Virtualize or Eliminate Investigator Meetings
Re-imagine the traditional PI meeting and convert it into a far more effective and insightful on-demand experience.
Deliver your protocol as an interactive learning experience that engages participants on-demand, evaluates confidence in conducting the study, and informs a more streamlined site initiation process.
Identify which sites are best equipped to succeed in performing the study, prioritize those sites for activation, and accelerate enrollment milestones.

Improve CRO Oversight
Ready helps you prevent study delays, by identifying strengths and weaknesses in your teams before the study begins.
Make more informed oversight and operational decisions that enable you to manage and invest resources more efficiently.
Then better equip and upskill your teams through automated engagement and knowledge retention programs to accelerate enrollment and prevent delays.

Streamline Site Initiation & Training
Transition site initiation visits into more scalable, consistent, and tailored on-demand training experiences using existing study content.
Convert study documents to interactive experiences that virtually equip site personnel with the knowledge and skills to effectively conduct your study.
Identify risks earlier, reduce delays and waste by identifying underperforming sites faster, proactively identify and address areas of confusion around critical study components sooner.
Want to reduce trial delays and costs?
arn how Ready enhances your ability to predict and improve study performance.