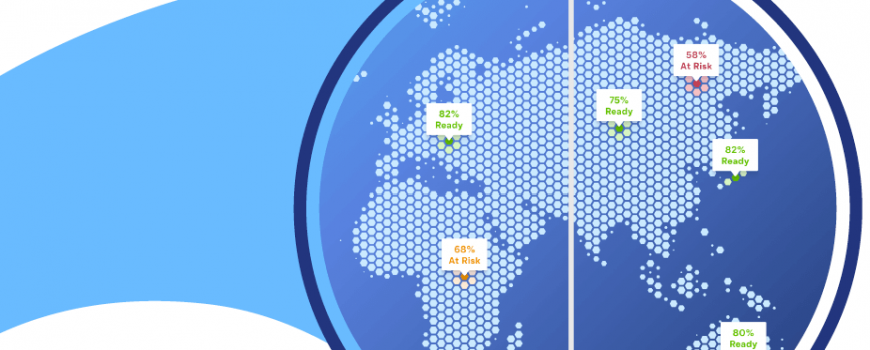
It should come as little surprise that the rise in decentralized trials (DCTs) and increase in study complexities have magnified performance demands across clinical trial teams.
 A recent survey by Ernst & Young found 50% of clinical trials will be hybrid or decentralized by 2024. This is welcome news for sponsors and especially patients as DCTs increase patient access and diversity. However, it also means more far reaching, differentiated tertiary endpoints to manage, monitor, train, and evaluate. Together, these changes increase the operational complexity and risk profile of future studies.
A recent survey by Ernst & Young found 50% of clinical trials will be hybrid or decentralized by 2024. This is welcome news for sponsors and especially patients as DCTs increase patient access and diversity. However, it also means more far reaching, differentiated tertiary endpoints to manage, monitor, train, and evaluate. Together, these changes increase the operational complexity and risk profile of future studies.
Home health staff, caregivers, and patients are now playing more active roles across the study. They are being asked to conduct new procedures and capture more data. This too magnifies risks. Why? PIs at individual sites no longer provide the same level of oversight and environmental control.
For Sponsors and CROs, preparing traditional sites and decentralized teams in a timely, effectively trained, and measurable manner has become a mission critical and increasingly difficult task. As a result, of the roughly 401,898 globally registered clinical studies currently in process, more than 90% will miss key milestones. Even worse, thousands will ultimately fail to meet primary endpoints due to the operational complexity and costly delays that could have been avoided.
Many of the challenges that lead to these delays could be identified far sooner and prevented. To more effectively manage the increased complexity, trial teams must identify the most likely risk areas before patients are enrolled and implement proactive strategies to address them. Thankfully, a growing number of trial sponsors are taking these steps.
The latest ArcheMedX White Paper: DCTs at Risk, or Ready? Assessing the Risks and Readiness of your next Clinical Trial explores these challenges and provides specific guidance for trial sponsors to mitigate risks as they advance clinical research in new and innovative ways.
Click here to download the DCTs at Risk, or Ready White Paper