Fresh Starts, Real Readiness: Turning Site Initiation into a Trial Success Multiplier
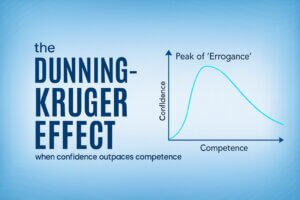
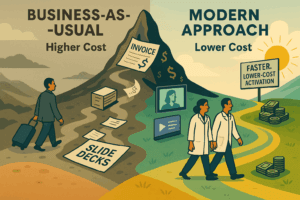
By leveraging the Fresh Start Effect at site initiation and shifting from passive training to demonstrated readiness, sponsors and CROs can turn trial launch into a powerful multiplier for early performance, compliance, and execution quality.