
77% of life sciences organizations want to improve how they identify which sites are ready to start a trial. Ready by ArcheMedX enables you to deliver a clinical trial protocol to a global audience within just days of getting started.
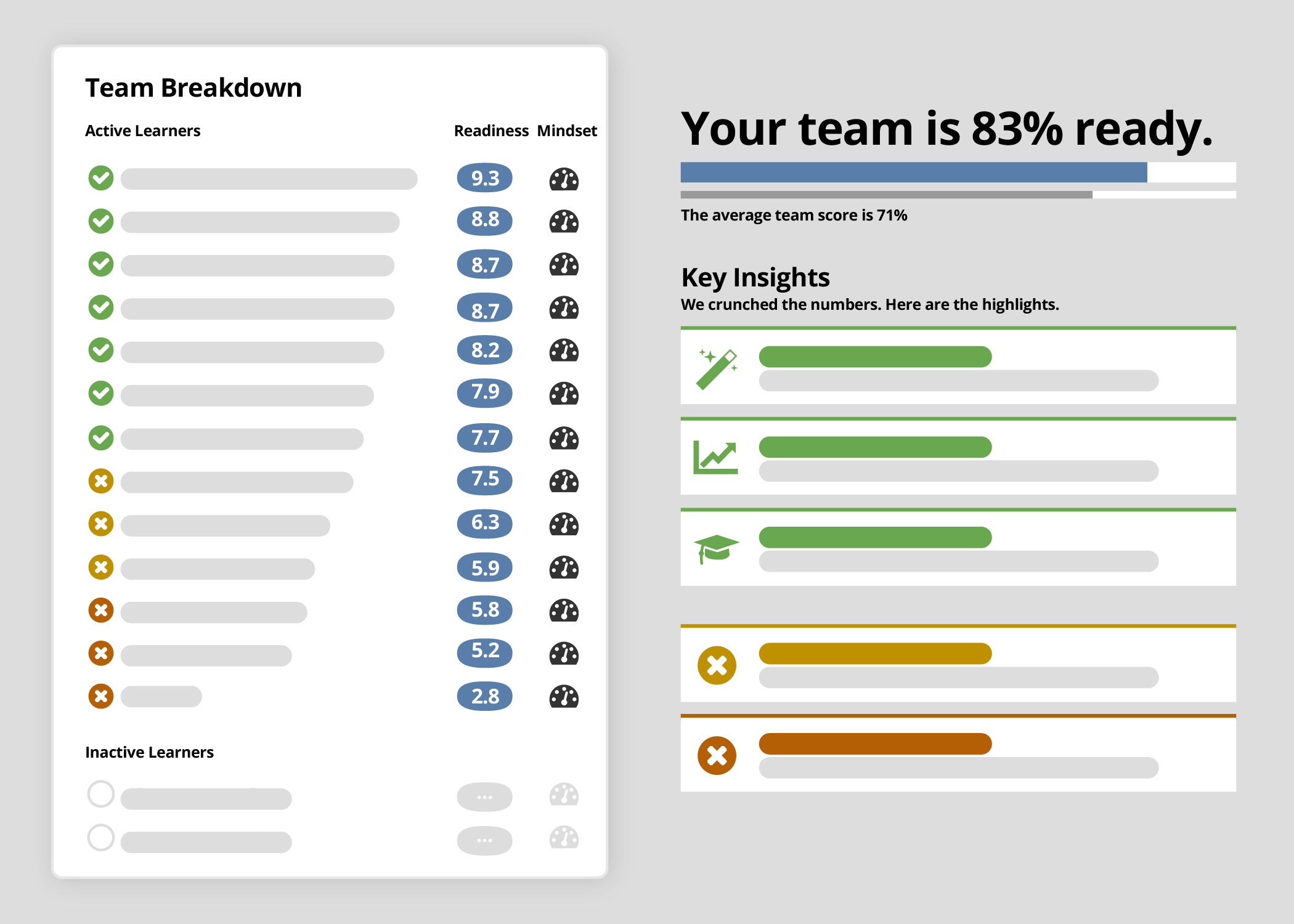
Powerful insights allow you to confidently predict who will succeed in conducting your clinical trial – and who needs more preparation.
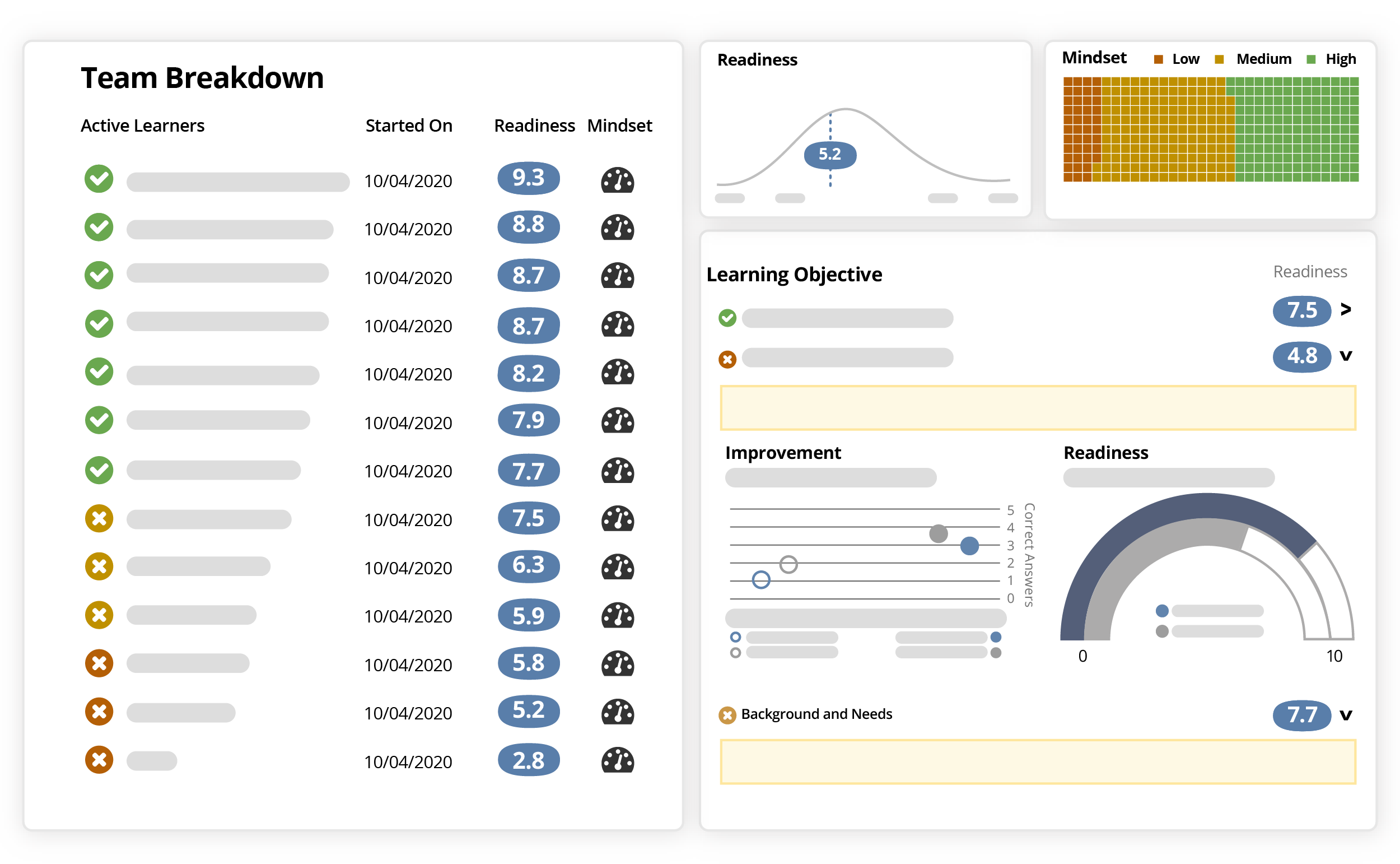
Complete your PI meeting with Ready in less time than it takes to decide on your agenda.
You know how expensive the in-person PI meeting can be. Delivering your meeting completely with Ready will save you tens of thousands on travel and hosting costs – and give you better results.
With unmatched analysis of participant knowledge and readiness to succeed with your trial, Ready lets you confidently measure understanding and predict how an individual will apply clinical concepts in real-world scenarios.
Eliminate the guesswork and activate sites confidently with Ready’s readiness scoring.
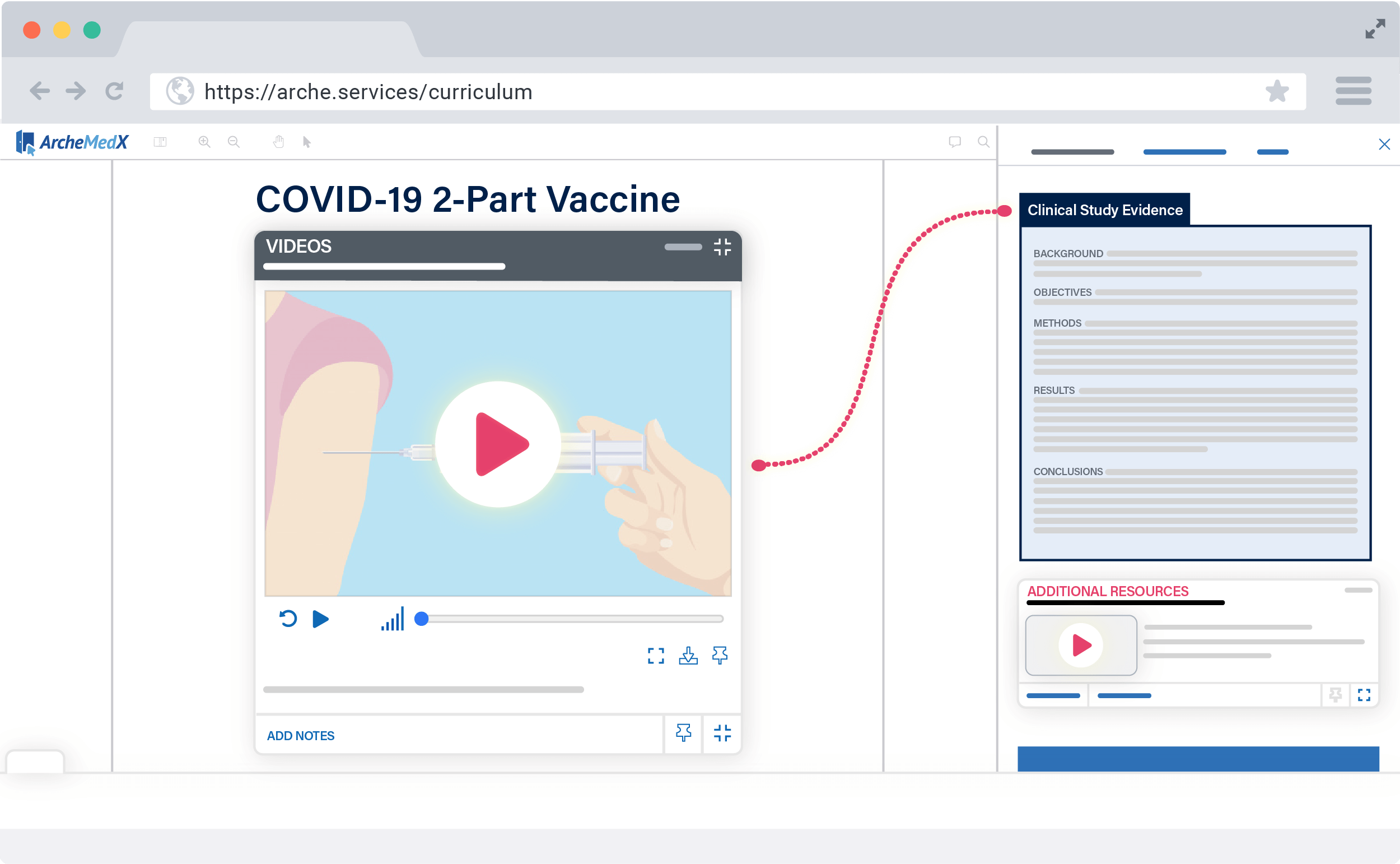
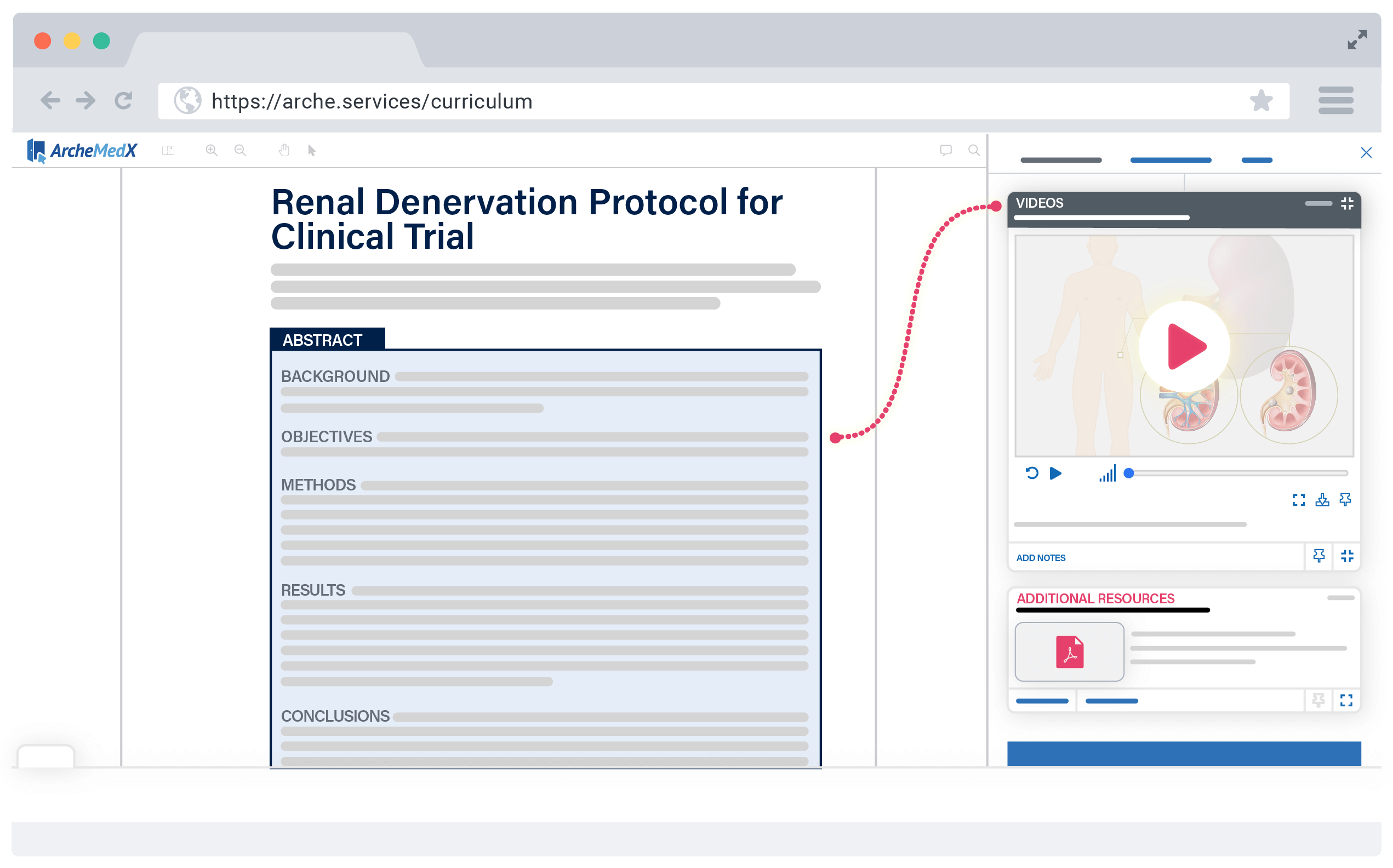
With Ready, you can deliver an interactive protocol, multimedia resources, and confidence-based assessments within days – to your entire global PI audience.
Ready’s intuitive interface guides you through creating an ideal learning experience for your participants, as well as helps you launch easily and securely to your audience.
Be live in just days, not weeks, with a PI meeting experience that’s so simple, your investigators will be able to focus on the protocol, not the process.
Built-in analysis ensures you confidently know which investigators truly understand your protocol, SOPs, and key objectives.
Enjoy knowing they’ll be better equipped to enroll eligible patients sooner, hit enrollment targets, and avoid screen failures. And prove the impact of your content and efforts with Ready’s powerful analysis and scoring data.
Before the COVID-19 pandemic hit, the investigator meeting was a non-debatable necessity in the beginnings of a clinical trial. Unfortunately, they also made up a huge budget line item – with the average price tag coming in close to $1 M USD for a global series of PI meetings.
But they’re off the table with the COVID pandemic – or are they? The fact is, you can pull off an effective, virtual PI meeting. Here’s how to make sure you do.
This content section link to the new pillar page, or could be a downloadable version of the pillar page.
Automate the engagement and upskilling of your team members who don’t need direct intervention.
Give your managers the insights to know where to invest their time, and where they can gain efficiencies. Focus on the team members who need 1:1 coaching the most.
Imagine saying this a year ago: “We’re planning a virtual investigator meeting.”
That simple statement would have sounded risky and provocative. It might have invited more than a few critics to challenge your plans.
Today, however, if you’re going to start a clinical trial, you’ll be among the minority if you’re not planning to deliver your investigator meeting remotely.
Get started today and see how fast, easy, and effective the virtual primary investigator meeting can be with Ready.