
Predict and improve clinical trial
site and team performance.
Uncover risks sooner. Eliminate delays.
Meet Ready, a Smarter Way to Accelerate Enrollment and Minimize Risks.
Ready by ArcheMedX transforms site qualification and site initiation to reveal which teams and sites are best prepared to successfully conduct the most critical aspects of your study
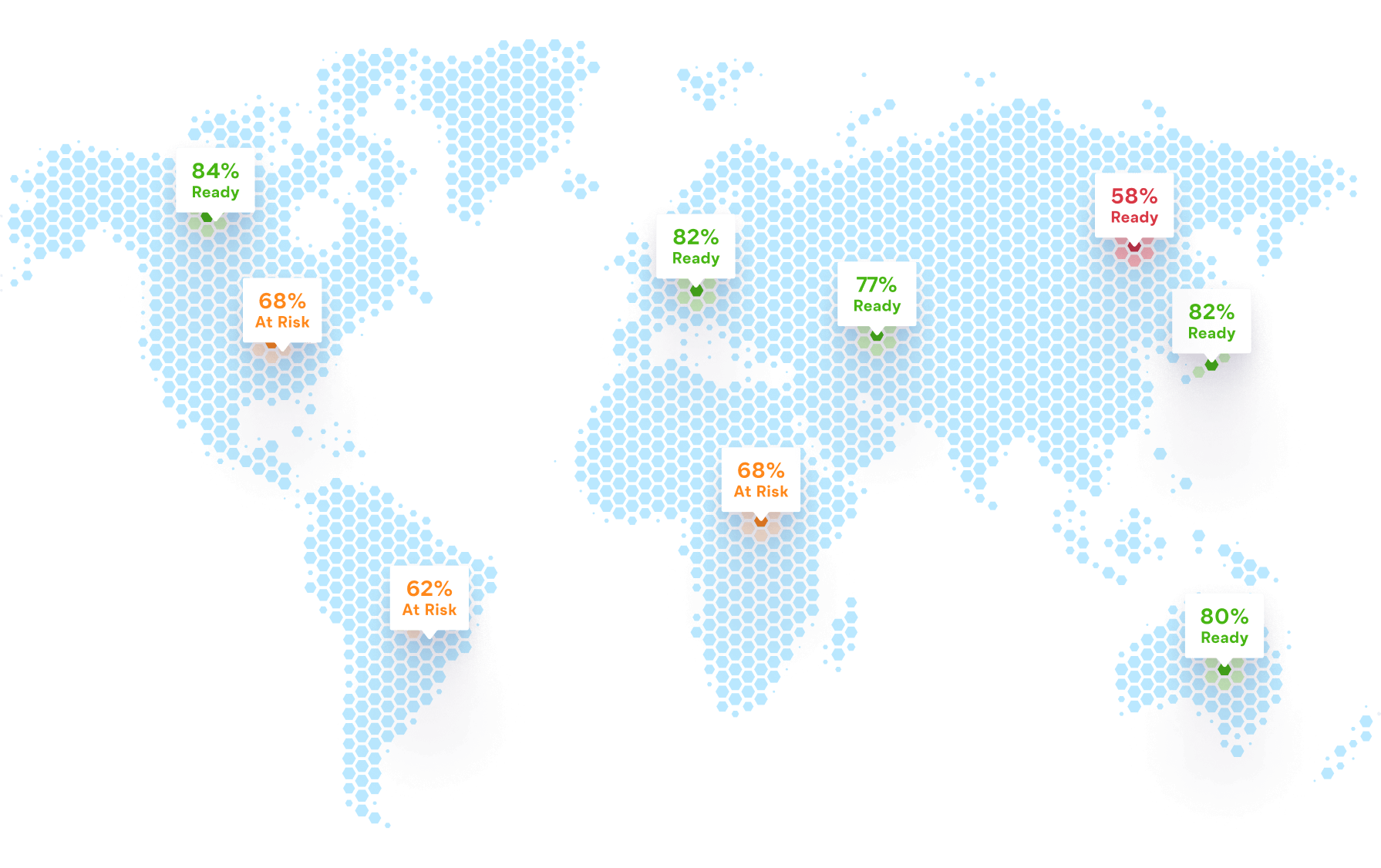
SELECT and prioritize the right sites
77% of trial sponsors and CROs want to improve how they identify which sites are ready to start a trial. Ready by ArcheMedX enables you to confidently predict who will succeed in conducting your clinical study – and who needs more preparation.
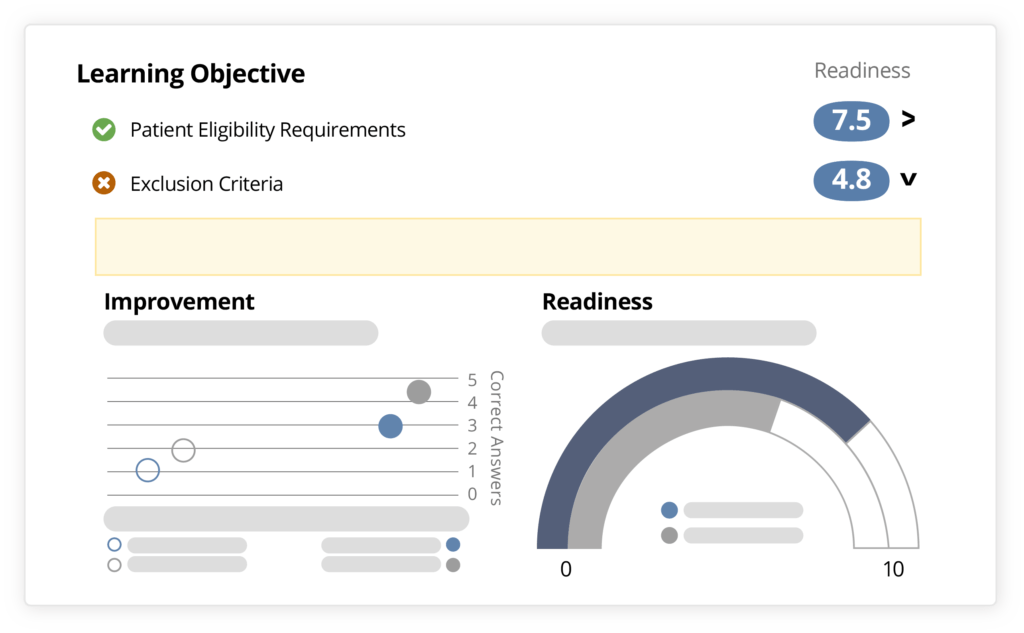
Using Ready’s benchmark data and analysis of individual site personnel's understanding of key study objectives, trial leaders can more effectively and rapidly assess and prioritize those sites and decentralized teams best equipped to accelerate enrollment and hit early milestones.
PREVENT COSTLY DELAYS – AT ANY STAGE
A whopping 85% of trials will experience a delay. Don’t be a statistic.
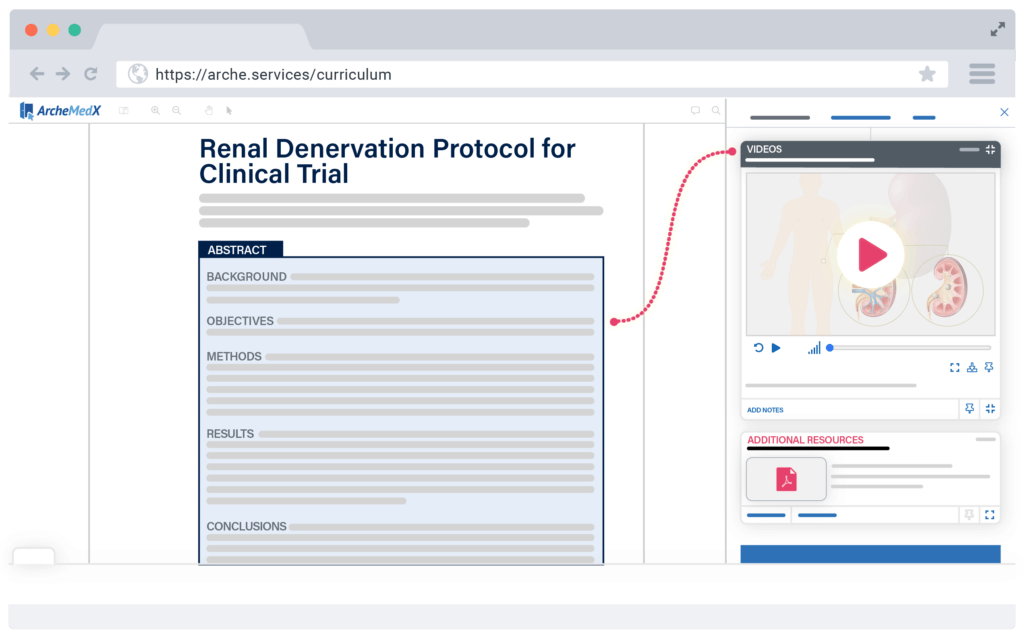
Most study delays are preventable with proper training and earlier identification of risks. Ready does both by converting your actual study documents into more interactive learning experiences that enhance a trial team member’s ability to consume and retain key study information.
Nudge individuals to focus on what is most critical for them in their role and deploy a variety of assessment models to more effectively measure their knowledge, confidence, and mindset. Then let Ready assess how confident they really are to apply the correct information during the study.
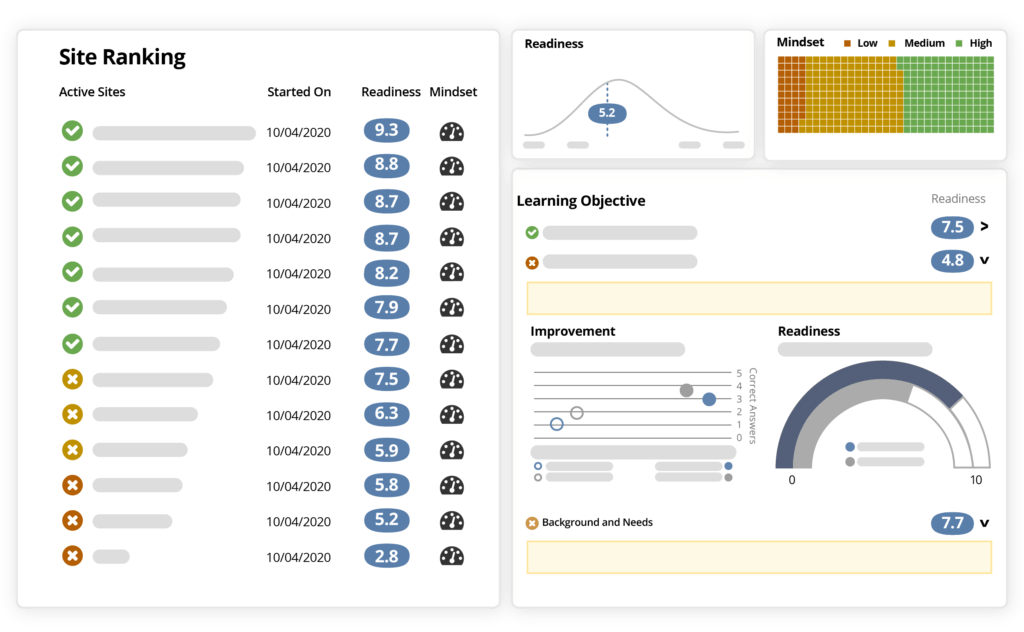
IMPROVE SITE and team PERFORMANCE
As much as 86% of trials don’t meet recruitment targets on time.
Ready helps you reinforce key study objectives, like inclusion/exclusion criteria, patient eligibility, IP and sample management, and other critical points. Then Ready evaluates each individual's knowledge and confidence against benchmark data to predict how effective sites and individuals will be in achieving those objectives.
Ready also reveals areas where your sites need additional preparation, so you can focus your time and resources only on the sites that need more help, and the topics that need more clarification.
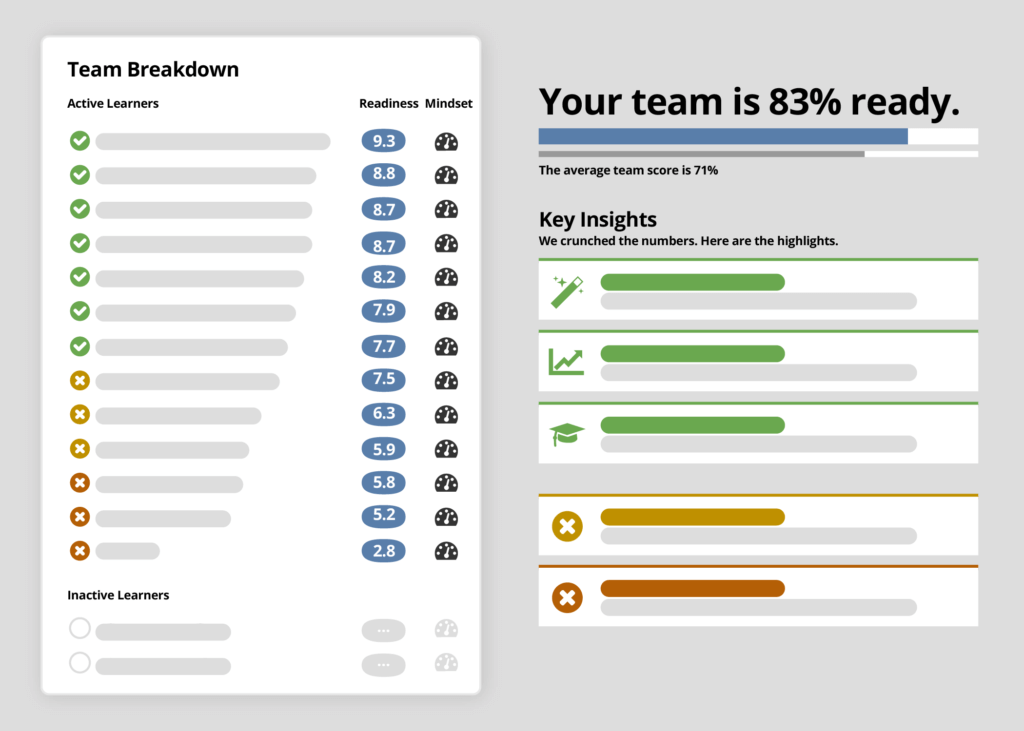
reduce the risk in decentralized trials
With decentralized trials increasing in adoption, the risks are magnified as home health staff, caregivers, and patients play more diverse roles across the study and in data collection. Ready enables trial sponsors to increase oversight and ensure decentralized teams are prepared for the remote challenges of today and the evolving digital future of tomorrow.
Use Ready to evaluate and improve each team member's understanding and confidence to perform their role and use Ready’s validation of individual readiness to prioritize trial activities and inform risk-based monitoring.
Reduce home visit costs while increasing adherence to your protocol.

CREATE MORE CAPABLE trial teams – AUTOMATICALLY
Once you know who is risk-prone, what is your plan of action? Ready can help you build and execute automated programs that prompt and engage your staff and decentralized teams to focus on identified areas of need.
Enhance consumption and understanding of amendments, best practices, and new content throughout your study’s duration. Then assess how likely sites and individuals will be in applying new information in practice.
Engage your teams in an ongoing way with Ready. Build an automated cadence of learning boosts to ensure content is mastered and key objectives are reinforced. Then measure and analyze their performance over time.
Why Should You Try Ready?
These Organizations Did – Check Out Their Results
Emerging Biotech Streamlines Site Initiation
By deploying Ready, an emerging biotech and mid-sized CRO re-imagined site initiation to convert the traditional PI meeting and SIVs into a tailored, on-demand training experience powered by Ready. As a result, the trial sponsor streamlined the overall initiation process and reduced their SIVs from 4 hours to 30 minutes.
Global CRO Transforms Clinical Delivery
A top five CRO dramatically improved its workforce performance management utilizing Ready to change the way clinical delivery managers and directors evaluated and equipped their project teams to more rapidly identify, upskill, and deploy the most capable staff on global clinical studies.
Research Center Reduces Staff Evaluation Time by 40%
A nationally recognized academic research center utilized Ready to accelerate on-boarding for new project members and accurately predict high and low performers. The program manager reported to leadership that using Ready would streamline staff evaluations, increase preparedness, and ultimately reduce time by 40-50%.
Want to See Similar Results?
Ready can help you transform areas of your clinical operations that are in need of a digital overhaul.
Let’s talk today and find out where you can improve your trial team performance.